The Week in Space and Physics: The Nobel Prize
On the Nobel Prize, the purest star yet known, hints of an exomoon, and a sign of life around Saturn.

This year, the Nobel Prize in Physics was awarded to a trio of quantum physicists. Their work, carried out in the 1980s, demonstrated how the strange effects of the quantum world can be scaled up, as the Nobel Committee put it, to something “big enough to be held in the hand”.
The quantum world, of course, is famously bizarre. But this weirdness is often easy to ignore: though individual particles may vanish and reappear, cross impenetrable barriers, or find themselves in contradicting states, this tends to average out when we look at systems of many particles. Big objects – tennis balls, humans, planets – generally behave themselves, and don’t mysteriously vanish or pass unexpectedly through walls.
Yet under the right circumstances, it is possible to make large objects act in quantum ways. The prize this year was awarded for experiments that showed this taking place in electrical circuits, and which demonstrated an effect called quantum tunnelling on a macroscopic scale.
This effect says that particles can, with some small probability, cross barriers that would normally be forbidden. As an example, one can imagine a ball rolling up a hill. If the ball has enough speed, it can crest the hill and start rolling down the other side. But if it doesn’t, the ball will instead stop and roll back down.
The other side of the hill, in this scenario, is forbidden to the ball. Classically this is all clear – the ball either has enough speed or it doesn’t. A slow ball will never find itself on the forbidden side. Quantum theory, however, offers another way to cross the hill. Instead of cresting the top, the ball may sometimes “tunnel” through the barrier, and thus reach the forbidden zone even if it doesn’t have enough energy to roll all the way to the top of the hill.
As strange as it sounds, the equations tell us this is possible even for large objects. In theory a tennis ball really could tunnel through a hill and spontaneously appear on the other side. But in reality, the probability of this happening is effectively zero. And if we increase the mass of the ball and allow the barrier to grow steeper, the odds will fall even further.
But for small particles – an electron, say, or a proton – facing low barriers, the odds become more favourable. Indeed, quantum tunnelling is used in some electronic chips, plays a vital role in nuclear fusion, and may even be the cause of occasional mutations in DNA. As bizarre as it is, quantum tunnelling on the microscopic scale is a well established phenomenon of our world.
This year’s laureates – John Clarke, Michel Devoret, and John Martinis – together experimented with ways to build electrical circuits that could exhibit this same quantum behaviour. To do this, they first made a system in which large numbers of individual particles were made to act together, effectively forming a single quantum state. They then showed this state truly followed quantum laws, and saw evidence of quantum tunnelling taking place.
At the time, Clarke said, this didn’t seem like work that might one day lead to a Nobel Prize. But the implications of it were broad. The circuits they built brought quantum behaviour into our macroscopic world, and allowed others to develop new technologies with them. Among these were quantum computers. Indeed, quantum circuits are often used as qubits, the basic units of information processed by these machines.
Quantum computing, of course, is still in its infancy. But if, or when, engineers can get them to work as expected, their ability to use quantum laws could greatly accelerate our ability to perform some calculations. That would be transformative; and if it happens, it will owe a lot to the work of Clarke, Devoret, and Martinis.
The Most Pristine Star Yet Known
Ask a chemist to define the term “metal”, and you might get back a long-winded explanation about conductivity and lustre, about electrons, and about positions on the periodic table of the elements. Ask an astronomer, and you’ll hear something far simpler. A metal, they will tell you, is any chemical element heavier than hydrogen or helium.
In the beginning, our universe contained very little metal. The Big Bang created a lot of hydrogen and a scattering of helium, and almost nothing heavier. This was the pristine state of the cosmos, and it was from this “pure” material that the first stars formed.
We know little about this first generation of stars. Models suggest they were enormous, perhaps ten thousand times the mass of the Sun, and that they burned brightly and died in spectacular explosions. In their hearts heavy elements were forged, and when they died they seeded the heavens with metals.
This first generation of stars thus began a long process of increasing metallicity in the cosmos. The process continues today – as stars like our Sun are born, burn, and die, they fuse together light elements to release energy and produce metals. From this, we can estimate the age of a star – the earlier it was born, the purer, or less metallic, the material it formed from.
So far, though, astronomers have found few traces of these actual first stars. They died long ago, and though they must have been bright, our telescopes have not yet picked up any trace of their ancient light. But we have found a handful of stars that may have formed from their ashes.
One example of this – and the most pristine star yet found – was reported in a recent study. It is called SDSS J0715-7334, and lies in the outer edge of the Milky Way, near a satellite galaxy called the Large Magellanic Cloud. By mass it is almost pure, and as a result the astronomers who studied it think it formed billions of years ago from the debris of a single first generation star.
It thus joins a growing collection of ancient and very pure stars known in our galaxy. It also complements observations of far-away and almost pristine galaxies detected by the James Webb Telescope. This star might be a survivor from those long-gone times. And it is, for now, the closest we have gotten to the first stars to light up the heavens.
An Exo-Moon Discovery?
There are now more than six thousand known planets in our galaxy. Many of them surely have moons – indeed, there are likely more moons out there than planets. But so far astronomers have not yet discovered even a single moon beyond the edge of the solar system.
That’s not necessarily a surprise. Moons are small and can be hard to see. Even today, we are finding new ones around Jupiter and Saturn, although many of these are, in truth, little more than captured asteroids. Yet as our telescopes improve, we should eventually begin finding moons around exoplanets.
If a recent study is right, traces of one might already have been seen around WASP-39b. This is a giant planet orbiting a star about seven hundred light-years away. In the past, clouds of sulphur dioxide have been detected in its atmosphere. Their origin remains uncertain, but the new study suggests they come from an unseen moon.
If so, this moon might look something like Io, the highly volcanic moon of Jupiter. As it orbits close to the gas giant, its core flexes under immense gravitational tides. This heats it and results in violent eruptions spewing out gases like sulphur dioxide. A similar process could be playing out around WASP-39b – and if so, there might be a moon awaiting discovery.
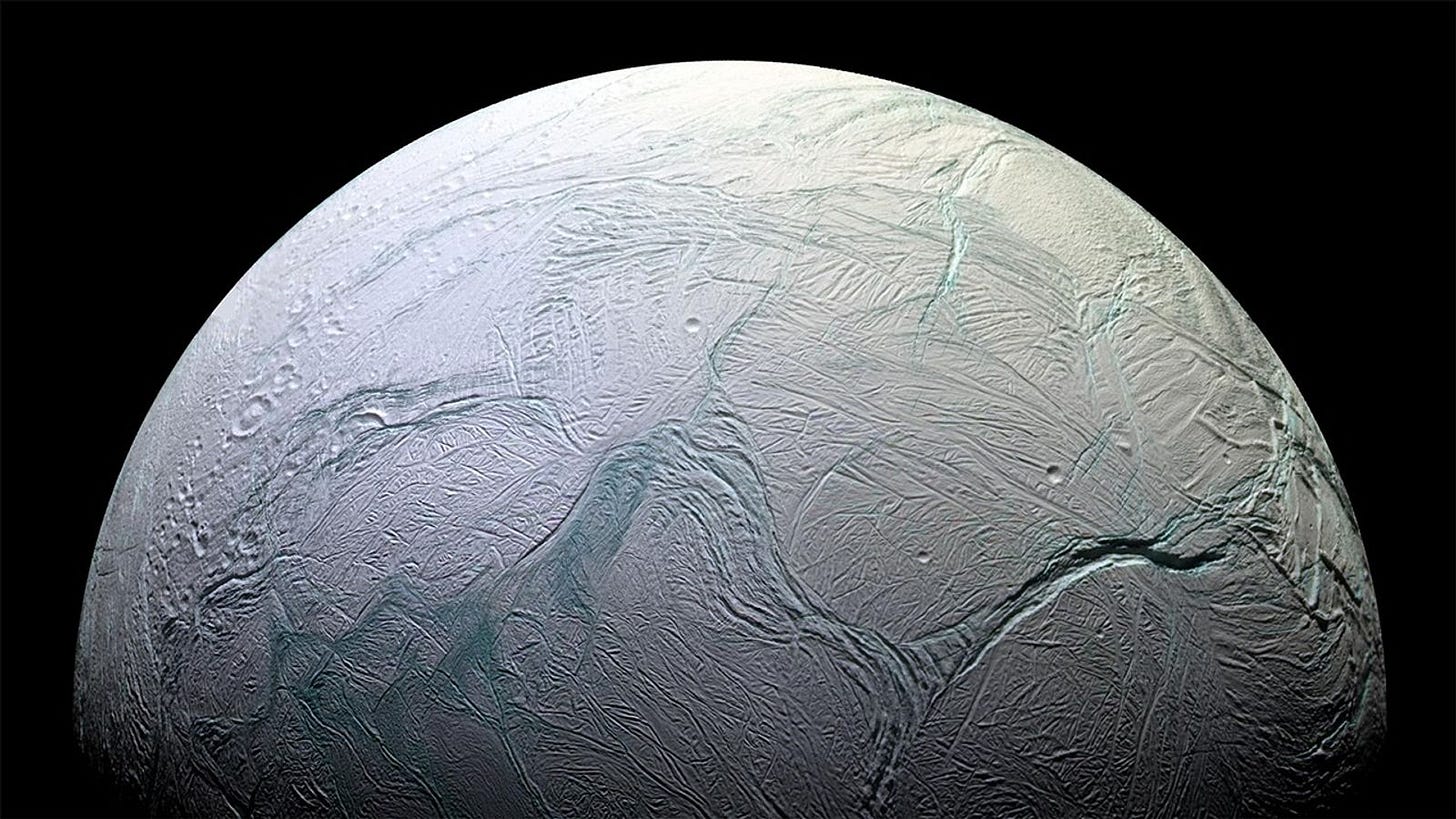
Hints of Life on Enceladus
Saturn’s moon Enceladus is fast becoming a top contender in the hunt for life beyond Earth. Although at first glance it appears to be little more than a frozen ball of ice, data gathered by the Cassini probe two decades ago revealed the presence of a liquid ocean hidden under its icy surface.
At times this ocean breaks free, erupting in towering plumes of salty water. On several occasions, Cassini flew through these plumes, gathering data about what they are made of. The probe’s instruments thus spotted traces of water, hydrocarbons, and organic compounds. Later studies have found signs of a tantalising mix of elements, including all those essential to the formation of life.
Now, a detailed analysis of one of Cassini’s flights over Enceladus has revealed signs of even more complex organic compounds. On Earth these chemicals are often associated with life – though, of course, we don’t yet know if that is the case on Enceladus. Yet their presence does tell us the moon’s oceans are chemically complex, and well worth further investigation.
Read More
The Myth and Mystery of The Tunguska Impact
Suddenly... the sky was split in two and high above the forest the w…
On Progress and Revolution in Physics
Speaking generally, we might say science progresses in two distinct ways.
How to Destroy a Space Station
For my paying subscribers there is a bonus article this week: Black Holes, Colliding Galaxies and a M…